Miranda Xie, 7 August 2024, first published by IAM
Precis: The CNIPA’s examination practice when it comes to targeted therapy drugs is tightening, with examiners increasingly inclined to reject claims. Against this backdrop, it is crucial for patent applicants to submit diverse examples to secure a satisfactory scope of protection and build a solid case.
Targeted cancer therapies involve agents that directly or indirectly attack a specific genetic biomarker found in a given cancer. Examples of targeted drugs include small molecules, antibodies, polypeptides, antibody-drug conjugates and nucleic acids, among others.
For newly discovered biomarkers associated with a certain cancer, it is typical to file for a patent application over targeted therapy drugs, apart from when it comes to diagnostic use. In China, claims of such pharmaceutical use are drafted as Swiss-type claims, which often read: “agents that [inhibit a target] in the preparation of a medicament for the treatment of [a certain disease].”
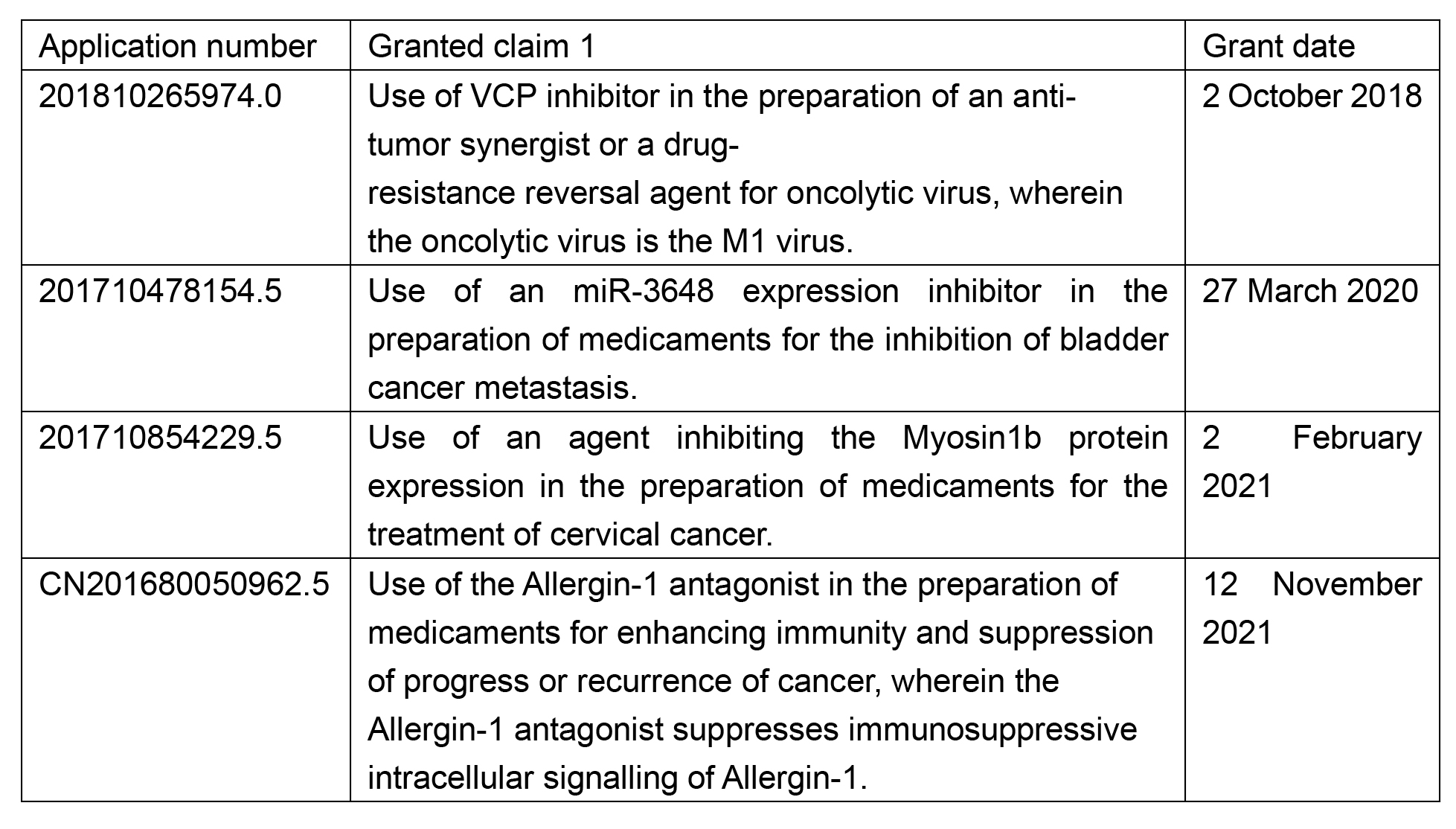
Over the years, the China National Intellectual Property Administration (CNIPA)’s examination practice on this issue has shifted. In most cases, the CNIPA used to allow the pharmaceutical use of biomarker-derived agents, which were drafted to cover a broad scope of protection, as long as the pharmaceutical effect of the target is new. This led to the smooth granting of patents in the last 10 years with biomarker-related features, broadly defined as ‘inhibitor’, ‘a(chǎn)ntagonist’ or ‘a(chǎn)gonist’. Although some were further defined with functional features, most had a reasonably satisfactory protection scope. See below for some examples.
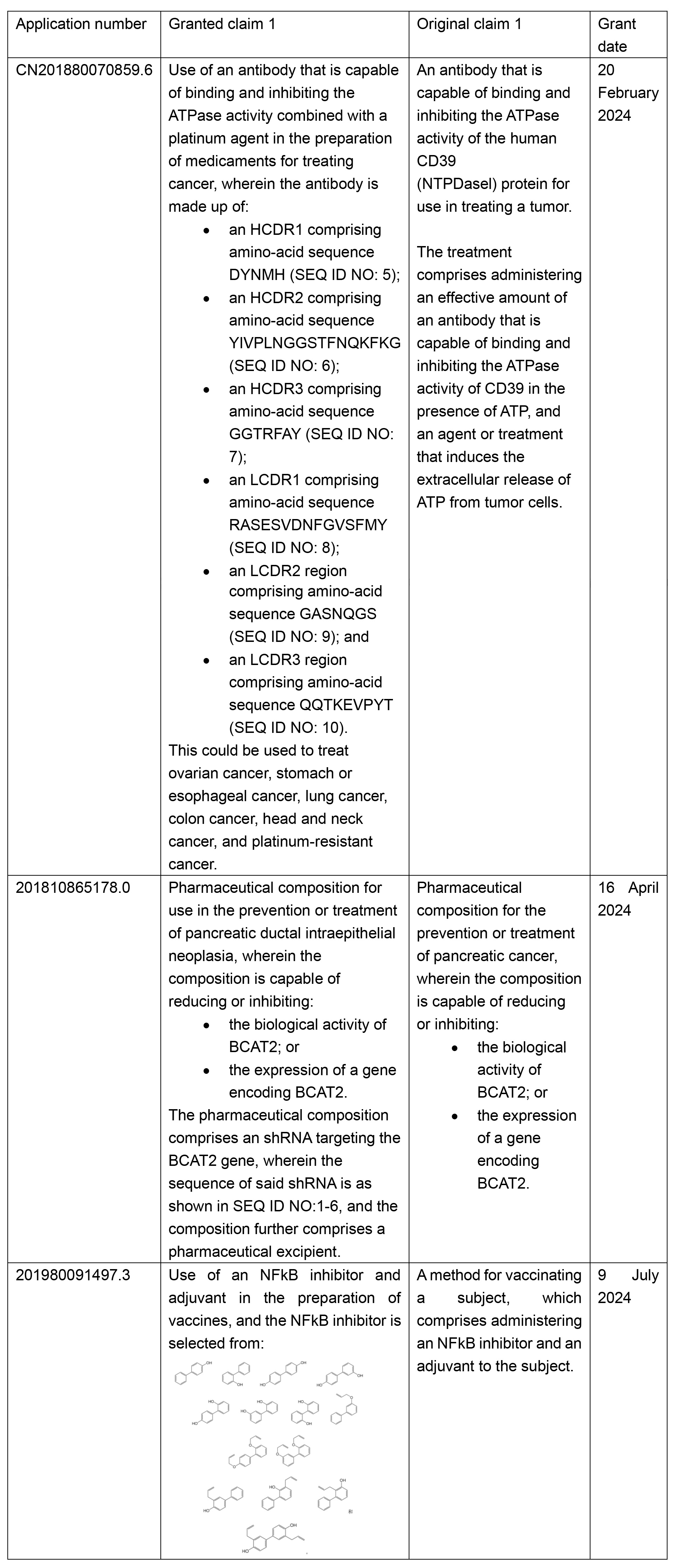
In the last two years, the examination criteria over targeted therapy drugs have been gradually tightened. In general, examiners are increasingly inclined to reject the claim on the ground that target-related features are devoid of support from the specification. See below for some recent examples of applications, which have been amended to overcome rejection.
On top of formal office actions, examiners are increasingly resorting to phone calls with patent attorneys to propose amendments that often further limit the claims.
In order to secure a satisfactory scope of protection, applicants seeking to patent drugs for biomarker-targeted therapy should submit diverse examples (eg, nucleic acid molecules, antibodies and small molecular) to build a solid case.
Further, if an applicant fails to secure a satisfactory scope of protection during the substantive examination process, it would be worth trying the reexamination procedure to reverse the initial decision, or to at least regain some lost ground. For example, in decision 1F422128, which was issued on 22 April 2023, the CNIPA’s reexamination board allowed for a more reasonable scope than the examiner in the substantive examination. Of course, applicants must take into account the breadth of the specification in assessing the viability of reexamination. For example, specifications with mere experimental data of small-molecular examples would be highly insufficient to support a reasonable scope.
Finally, if the pharmaceutical use of a targeted drug cannot be granted with satisfactory scope, another option is to claim for a drug screening method.



